Choosing the wrong electrode materials can lead to system failure and wasted costs. Unsure which materials fit your electrochemical process? The choice depends heavily on your specific application and operating environment.
Anodes require corrosion-resistant materials like MMO-coated titanium, while cathodes need good conductivity, with stainless steel and nickel being common choices. The best material is always determined by the specific electrochemical application, electrolyte, and desired lifespan.

Coming from Baoji, a global hub for titanium production, I’ve spent years working with metal-based electrodes—especially titanium anodes—for electrochemical applications. One common question clients ask me is: What materials are best for anode and cathode use? My answer always starts with: It depends on your application and electrolyte. When you understand the materials—and the electrochemistry behind them—you can design far more reliable and efficient systems. And that’s where we add value.
What are the materials used for anode and cathode?
Are you struggling to select the right materials for your electrodes? Using the wrong one can cause corrosion or poor performance. It’s crucial to know which materials are suited for the demanding roles of anode and cathode.
Common anode materials include MMO-coated titanium, platinized titanium, and lead dioxide. For cathodes, materials like stainless steel, nickel, and graphite are frequently used due to their conductivity and stability in reductive environments.
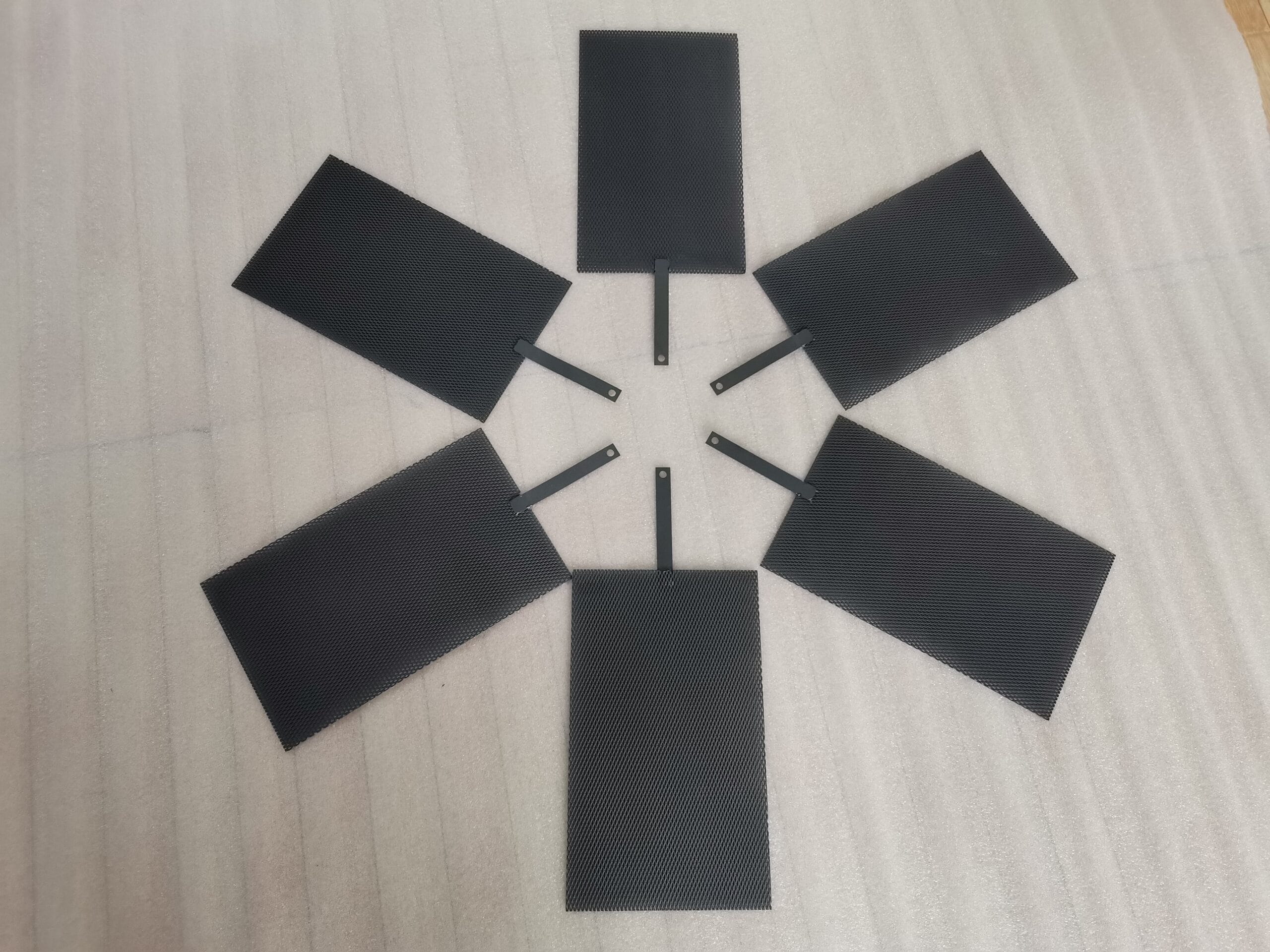
The selection of anode and cathode materials is critical for the efficiency and longevity of any electrochemical cell. The anode, where oxidation occurs, faces a highly corrosive environment and requires robust materials. The cathode, where reduction occurs, has different demands. Over my years helping clients, I’ve seen that the best choices balance performance, cost, and lifespan. For instance, in anodizing processes, titanium is used as the anode while materials like aluminum or stainless steel serve as the cathode . Chemical titanium is widely used for making both anodes and cathodes in the electroplating industry due to its excellent resistance to corrosion .
Comparing Common Electrode Materials
Here is a breakdown of common materials I recommend to my clients:
| Electrode | Material | Key Characteristics & Common Uses |
|---|---|---|
| Anode | MMO-Coated Titanium | Excellent corrosion resistance and catalytic activity. Used in water treatment, chlorine generation, and electroplating. |
| Anode | Platinized Titanium | High performance and efficiency, but more expensive. Used for precision electrolysis. |
| Anode | Lead Dioxide (PbO₂) | Strong oxidizing power. Use is declining due to environmental and health concerns (toxicity). |
| Cathode | Stainless Steel | A cost-effective and durable choice for many aqueous systems. Can be used as a cathode when anodizing titanium. |
| Cathode | Nickel | Excellent performance in alkaline environments, such as in alkaline water electrolysis. |
| Cathode | Graphite | Chemically stable and affordable. However, it can be fragile and is less conductive than metals. |
Can graphite be used as a cathode?
Considering graphite for your cathode but worried about its limitations? Its brittleness and lower conductivity compared to metals are valid concerns. So, is it a practical choice for your application?
Yes, graphite can be used as a cathode. It is chemically stable and relatively inexpensive, making it a viable option in certain electrochemical applications, though it is more fragile and less conductive than metallic cathodes.
Graphite is a well-known material in electrochemistry and can certainly be used as a cathode. Its primary advantages are its chemical inertness in many solutions and its low cost, which makes it an attractive option for a variety of applications, from batteries to some electrolysis processes. In my experience, while graphite is functional, it’s often a trade-off. For instance, when I work with clients in demanding industries like chemical processing or marine applications, they typically prioritize durability and performance, which often leads them away from graphite. Materials like titanium offer superior durability and corrosion resistance, which reduces maintenance and ensures a longer lifespan .
Graphite vs. Metallic Cathodes
When a client considers graphite, I always encourage them to weigh its properties against those of metals like stainless steel or nickel.
- Conductivity: Graphite is conductive, but less so than most metals. In high-current applications, this can lead to greater energy loss.
- Durability: The biggest drawback of graphite is its brittleness. It can easily chip or break, especially in industrial environments with vibrations or mechanical stress. Metals like stainless steel or titanium are far more durable and resistant to wear .
- Purity: Graphite electrodes can sometimes shed small particles into the electrolyte, which can be a problem in processes requiring high purity.
- Cost: Graphite’s main advantage is its low price point. However, when you factor in a shorter lifespan and potential replacement costs, a more durable metal cathode can offer a lower total cost of ownership over time.
For these reasons, while graphite has its place, many industrial processes benefit from the robustness of metallic cathodes.
What is an example of a cathode and anode?
Need a real-world example to understand cathodes and anodes better? It can be confusing without a concrete illustration. Let’s look at a common and important industrial process: anodizing titanium.
A clear example is the anodizing of titanium, where the titanium part itself acts as the anode, and a sheet of stainless steel or aluminum functions as the cathode.

A great, practical example of an anode and cathode at work is in the process of anodizing titanium, a surface treatment I’m very familiar with from my work in Baoji. Anodizing is used to enhance the appearance, wear resistance, and corrosion resistance of titanium parts . In this process, the roles of the anode and cathode are very clearly defined, and it perfectly illustrates how different materials are chosen for each. The setup involves submerging both the anode and cathode in an electrolyte, such as a weak phosphoric acid solution, and applying a voltage .
The Roles in Titanium Anodizing
- The Anode: The anode is the workpiece itself—the piece of titanium or titanium alloy that you want to treat . At the anode, an electrochemical reaction is forced to occur. Under the applied voltage, the surface of the titanium oxidizes, forming a durable and protective layer of titanium oxide. By precisely controlling the voltage, you can even change the thickness of this oxide layer, which results in different colors on the surface.
- The Cathode: The cathode in this process is typically a sheet of a material like stainless steel or aluminum . The cathode completes the electrical circuit. Here, the reduction reaction takes place—in an aqueous electrolyte, this is typically the evolution of hydrogen gas. The cathode material is chosen because it is conductive and stable in the electrolyte, but it doesn’t need the extreme corrosion resistance of the anode because it is not being oxidized.
This example clearly shows the different functions: the anode is the material being actively changed (oxidized), while the cathode serves to complete the circuit and facilitate the corresponding reduction reaction.
What are the best anode and cathode materials?
Are you searching for the absolute "best" materials for your anode and cathode? This pursuit can be frustrating because there is no single answer. The optimal choice is always relative to the specific electrochemical system.
The best anode is often MMO-coated titanium for its durability and efficiency, while the best cathode is frequently nickel or stainless steel. However, the "best" materials depend entirely on the application’s electrolyte, cost, and desired lifespan.

From my years of experience, the question of the "best" materials is one I hear almost daily. The truth is, the "best" is what is most effective and economical for your specific context. A material that is perfect for a small lab experiment may be completely unsuitable for a large-scale industrial plant. The key factors I discuss with my clients are always the chemical environment, the required operational life, and the budget. For example, in the chemical industry, materials that can withstand harsh acids and high pressures are essential. This is where titanium alloys excel, offering exceptional corrosion resistance and high strength .
Selecting the Optimal Electrodes
To determine the best materials, we must consider the specific demands of the process.
- Best Anode Material: For a wide range of applications, from water treatment to electroplating, I consistently recommend titanium with a Mixed Metal Oxide (MMO) coating. It offers an unparalleled combination of corrosion resistance, durability, and high catalytic efficiency . While platinized titanium offers slightly higher performance in some cases, its high cost limits its use. For most industrial duties, MMO-coated titanium provides the best balance of performance and long-term value.
- Best Cathode Material: For cathodes, the choice is often between stainless steel and nickel. Stainless steel is a fantastic, all-around, cost-effective choice for many neutral or acidic aqueous solutions. It’s durable and reliable. For alkaline environments, such as in industrial-scale alkaline water electrolysis, nickel is often the superior choice due to its excellent stability and catalytic activity for hydrogen evolution.
Ultimately, combining a high-performance anode like MMO-coated titanium with a robust, cost-effective cathode like stainless steel often creates the most reliable and economically sound system for many industrial applications.
Conclusion
Choosing the right anode and cathode is crucial for performance. Coated titanium is often best for anodes, while stainless steel or nickel are top choices for cathodes, depending on your specific application.






