Watching valuable steel structures like boat hulls or pipelines rust away is a costly problem. This decay can lead to catastrophic failure, requiring expensive repairs and significant downtime for your operations.
A sacrificial anode prevents corrosion by being made of a more electrochemically active metal. When connected to the structure you want to protect, the anode corrodes preferentially, or "sacrifices" itself, while the main structure remains intact.

Coming from Baoji, China’s titanium valley, and working in the metal electrode field for years, I’ve often helped clients compare sacrificial anodes with advanced titanium MMO anodes. Many first ask: “How exactly does a sacrificial anode stop corrosion?” The principle is simple: a more active metal corrodes in place of the structure you want to protect. Over the years, I’ve learned that the key is not just knowing how sacrificial anodes work, but helping customers choose the right protection strategy—whether it’s a consumable metal or a durable titanium system.
How does a sacrificial anode prevent corrosion?
It seems strange that attaching one piece of metal can stop another from rusting. This simple trick feels almost like magic, but it is based on fundamental electrochemical principles that are incredibly reliable.
A sacrificial anode works by creating a galvanic cell with the metal it protects. Because the anode is more reactive, it becomes the active site for corrosion, releasing electrons that protect the less reactive metal (like steel) from oxidizing.
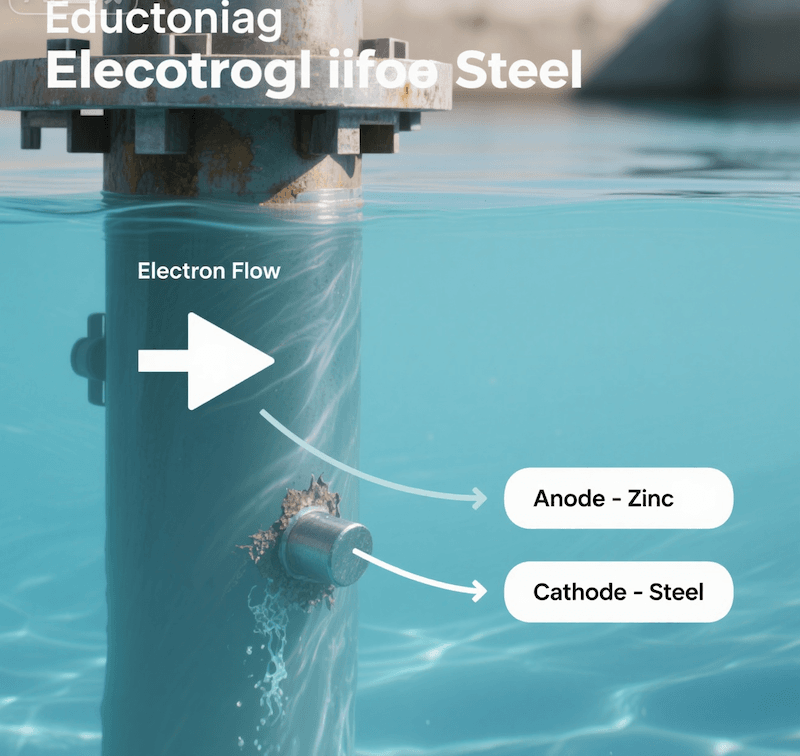
When you have two different metals connected in an electrolyte, like seawater, they form a small battery. The metal with a more negative electrochemical potential will always act as the anode and corrode first. By intentionally attaching a block of zinc or aluminum to a steel ship hull, you are directing the corrosion to happen on the zinc block. The zinc willingly gives up its electrons, which flow to the steel, preventing the iron in the steel from losing its own electrons and turning into rust. This is a very practical and effective method for protecting large steel structures. While this method is excellent, it has limitations. For applications demanding long life and stability without constant replacement, I often guide my clients toward solutions using MMO-coated titanium anodes. These anodes are not sacrificial; they use an external power source to provide protection and have an extremely long lifespan due to titanium’s exceptional corrosion resistancearxiv.org/pdf/2504.08794) resistance and durability [4][5][12].
What are the advantages of sacrificial anode technique?
You need a reliable way to protect your assets from corrosion, but the options seem complex. You are looking for a straightforward, proven method that does not require a complicated setup or power source.
The main advantages of using sacrificial anodes are their simplicity, low cost, and ease of installation. They do not require any external power source and provide reliable protection, making them ideal for many applications.
[image placeholder]
The beauty of the sacrificial anode technique lies in its simplicity. It’s a self-contained, passive system. You don’t need wires, power supplies, or complex monitoring equipment. This makes it a go-to solution for protecting isolated structures like buried pipelines or standalone water heaters. It’s a "set it and forget it" solution, at least for a while. However, the term "sacrificial" is key—they are designed to be consumed and must be inspected and replaced regularly. This leads to long-term maintenance costs [6]. This is where the contrast with impressed current cathodic protection (ICCP) systems using titanium anodes becomes clear. While the initial setup for an ICCP system is more complex, the anodes themselves are not consumed. A well-engineered MMO-coated titanium anode can last for many years, offering significantly lower maintenance costs and more consistent protection over its lifespan [4][6]. The durability and longevity of titanium make it the superior choice for large-scale or critical infrastructure projects [3][12].
How effective are sacrificial anodes?
You are considering sacrificial anodes but are worried about their reliability. Will they provide enough protection for your valuable equipment, or is it a half-measure that could still lead to costly corrosion damage?
Sacrificial anodes are very effective, provided they are correctly selected and installed. The level of protection depends on the anode material, its size, and the conductivity of the environment. In the right conditions, they completely stop corrosion on the protected structure.

The effectiveness of a sacrificial anode system comes down to good engineering. You have to match the anode material to the metal you’re protecting and the environment it’s in. For example, zinc works great in saltwater, but in freshwater, it can develop a passive film that stops it from working. Magnesium is more active and is often used in soil or freshwater. The system’s effectiveness also depends on having enough anode material to last between maintenance periods. When designed correctly, they are extremely reliable. But their effectiveness fades as the anode is consumed. For applications that require unwavering protection over a very long time, such as offshore platforms or large chemical processing vessels, the stability of an MMO-titanium anode is unmatched [4][5]. Because they are powered externally and made from highly corrosion-resistant titanium, their performance does not degrade over time, ensuring consistent and reliable protection for decades [1][7].
Does sacrificial protection prevent rust?
Rust is the enemy of any steel structure, and you need a surefire way to prevent it. You’ve heard about sacrificial protection but want to know if it truly stops rust from forming.
Yes, sacrificial protection directly prevents rust. Rust is the result of steel corroding (oxidizing). By forcing a more active metal to corrode instead, the sacrificial anode keeps the steel electrochemically protected, stopping the oxidation process that causes rust.

This is the entire point of the system. Rust, or iron oxide, forms when iron atoms in steel lose electrons. Sacrificial protection works by supplying a constant stream of electrons to the steel from the corroding anode (like zinc or aluminum). This electron supply satisfies the steel’s electrochemical needs and keeps it in a "reduced" or non-rusting state. The steel becomes the cathode in the electrochemical cell, and cathodes do not corrode. So, as long as the sacrificial anode is active and has material left to give, your steel structure will remain rust-free [1][7]. This is a powerful concept. However, remember that the anode is always being consumed. An alternative I work with is using inert anodes, like those made from titanium. These anodes, used in ICCP systems, also prevent rust by supplying a protective current, but they achieve this without corroding themselves. This ensures a permanent, rust-free solution with minimal need for replacement, highlighting the benefits of titanium’s superior corrosion resistancearxiv.org/pdf/2504.08794) resistance and longevity [9][12].
Conclusion
Sacrificial anodes are a simple, effective way to prevent rust by corroding in place of a protected structure. They are ideal for many applications but require eventual replacement as they are consumed.






