Watching your expensive anode parts degrade and fail is a costly problem. This unexpected corrosion can halt your production line, compromise product quality, and lead to expensive, unplanned maintenance.
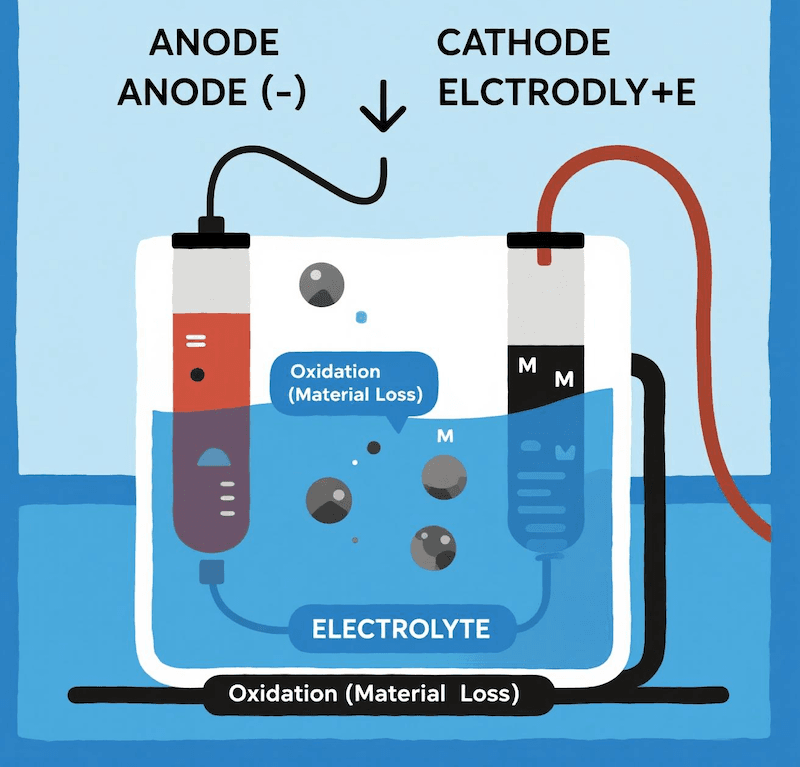
Corrosion is fundamentally an oxidation process. In any electrochemical cell, the anode is, by definition, the electrode where oxidation—the loss of electrons from a material—occurs. This loss of material is what we observe as corrosion.
Working at the heart of China’s titanium industry in Baoji, and specializing in custom titanium anode solutions/www.arxiv.org/pdf/2411.16741v2) solutions, I’ve often had to explain this basic but critical question to clients. The short answer is: Corrosion is oxidation, and oxidation happens at the anode. Understanding this isn’t just theory; it’s what I help my clients manage every day. With the right design, material, and operating conditions, you can turn corrosion into a controlled, useful process, not a threat.
Why does corrosion occur at the anode?
It is frustrating when you see your anode corroding. You might think the material is faulty or low-quality. The truth is that this process is a fundamental law of electrochemistry at work.
Corrosion occurs at the anode because it is the location where metal atoms lose electrons and transform into positively charged ions. These ions then dissolve into the surrounding electrolyte, which we see as the physical degradation or corrosion of the metal.

In any electrochemical system, there are two electrodes: an anode and a cathode. The anode‘s job is to give up electrons. When a metal atom at the anode‘s surface loses an electron, it is no longer a stable metal atom. It becomes a metal ion and can float away into the liquid solution (the electrolyte). This continuous loss of metal atoms from the surface is the very definition of corrosion. For example, in a system using a steel anode, the iron atoms (Fe) at the surface oxidize to become iron ions (Fe²⁺). This is why the anode appears to be eaten away over time. However, some materials are far more resistant to this process. My work with titanium focuses on its natural ability to resist corrosion due to a stable, protective oxide layer that forms on its surface [1][4][9]. This is why we use it as a base for building long-lasting anodes.
The Role of the Anode
- Electron Source: The anode serves as the source of electrons for the electrochemical circuit.
- Oxidation Site: It is the site where the oxidation reaction takes place. This is an unavoidable part of how the cell functions.
- Material Loss: For many common metals, this oxidation process results in the physical loss of material, which is what we call corrosion. In some cases, like the anodizing of titanium, this process is intentionally controlled to build a stronger, more corrosion-resistant oxide layer on the surface.
Why does oxidation always occur at the anode?
The rule that "oxidation occurs at the anode" can seem arbitrary. This confusion might lead you to misdiagnose problems in your electrochemical setup, costing you time and effort in troubleshooting.
By universal scientific definition, the anode is the electrode where oxidation happens. The term "anode" is assigned to the electrode that loses electrons. A simple mnemonic to remember this is "An Ox" (Anode = Oxidation).

This rule isn’t random; it’s a fundamental convention in chemistry and physics that helps everyone speak the same language. Think of it like defining "north" and "south." Once we agree on the terms, we can build reliable systems. In an electrochemical cell, there is a flow of electrons. The electrode from which electrons flow out is called the anode, and the process of losing electrons is called oxidation. The electrode that receives electrons is the cathode, and the process of gaining electrons is reduction ("Red Cat" for Reduction at Cathode). In the systems my clients use for electroplating or water treatment, an external power supply actively pulls electrons away from the anode. This forces oxidation to occur at the anode. My job is to provide anodes, like MMO-coated titanium, that can withstand this forced oxidation without degrading themselves.
Defining the Roles
Here is a simple table to keep the terms straight:
| Term | Role | Process | Mnemonic |
|---|---|---|---|
| Anode | Electrode where electrons are lost | Oxidation | An Ox (Anode-Oxidation) |
| Cathode | Electrode where electrons are gained | Reduction | Red Cat (Reduction-Cathode) |
Why corrosion occurs at anode and corrosion product deposits in between anode and cathode?
You see rust or other gunk forming, not just on the anode but floating in your solution. This contamination can ruin your electrolyte bath and compromise the quality of your final product.
Corrosion dissolves the anode into positive metal ions. These ions then travel into the electrolyte, where they can react with other negative ions (like hydroxide) to form insoluble compounds. This new compound is the corrosion product that precipitates and deposits as solid gunk.

This is a two-step process that I often explain to customers who work with less stable anodes](https://www.arxiv.org/pdf/2411.16741v2)s. First, corrosion happens at the anode surface. For example, an iron anode corrodes, releasing iron ions (Fe²⁺) into the electrolyte. Second, these newly freed ions don’t just disappear. They are now floating in the solution and are chemically reactive. In a water-based solution, they will quickly find negatively charged hydroxide ions (OH⁻) and combine with them to form iron hydroxide, Fe(OH)₂. This compound is not soluble in water, so it appears as a solid particle, which we see as rust or sludge. This is a huge problem in processes that require high purity. That’s why many of my clients are switching to stable anodes](https://www.arxiv.org/pdf/2411.16741v2)s like MMO-coated titanium. These anodes are designed to resist corrosion themselves [3][4][5]. Instead of the titanium dissolving, they drive the oxidation of other substances in the electrolyte, keeping the solution clean from unwanted anode corrosion products [11][12].
The Journey of a Corrosion Product
- Oxidation: An atom on the anode surface loses electrons (e.g., Fe → Fe²⁺ + 2e⁻).
- Dissolution: The newly formed positive metal ion (Fe²⁺) leaves the anode and enters the electrolyte.
- Precipitation: The metal ion reacts with negative ions in the solution (e.g., Fe²⁺ + 2OH⁻ → Fe(OH)₂) to form a solid corrosion product.
Why does galvanic corrosion occur?
You connect two different metals in a moist environment, and suddenly one of them starts rusting away fast. This unexpected and rapid corrosion can cause a critical part to fail without warning.
Galvanic corrosion is an electrochemical process that happens when two different metals are in electrical contact within an electrolyte. The more chemically reactive metal becomes the anode and corrodes at an accelerated rate, sacrificing itself to protect the less reactive metal.
This is a very common type of corrosion, and it’s driven by the natural voltage difference between two dissimilar metals. Every metal has a different tendency to give up its electrons. When you connect a more reactive metal (like zinc) to a less reactive one (like steel) in the presence of an electrolyte like seawater, you create a small battery. The zinc willingly becomes the anode and starts to corrode, protecting the steel cathode from rust. This is the principle behind using "sacrificial anodes](https://www.arxiv.org/pdf/2411.16741v2)s" on ship hulls. From my perspective as a titanium expert, this is a critical concept. Titanium is very noble, or non-reactive, due to its protective oxide layer [3][4][9]. If you bolt a steel part directly to titanium in a corrosive environment, the steel will become the anode and corrode very quickly to protect the titanium. Understanding and managing these galvanic pairs is essential for designing durable systems.
The Galvanic Series
To predict which metal will corrode, engineers use a chart called the galvanic series. Metals at the top are more "anodic" or reactive, while metals at the bottom are more "cathodic" or noble.
| Reactivity | Metal Example | Role in a Couple |
|---|---|---|
| More Reactive (Anodic) | Magnesium, Zinc | Corrodes (Sacrifices itself) |
| Medium Reactivity | Aluminum, Steel | Can be either, depending on the other metal |
| Less Reactive (Cathodic/Noble) | Stainless Steel, Titanium | Is Protected |
When you connect two metals from this list, the one higher up will corrode to protect the one lower down.
Conclusion
Corrosion is simply oxidation occurring at the anode. Understanding this fundamental rule allows you to manage it, either by using sacrificial anodes](https://www.arxiv.org/pdf/2411.16741v2)s or by choosing stable, corrosion-resistant materials like coated titanium.






